- Smallest Particle Of An Element Or Compound
- Smallest Particle Of An Element That Retains Its Properties
- Smallest Particle Of An Element That Maintains The Properties Of That Element
An atom is the smallest unit of ordinary matter that forms a chemical element.Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects. Definition of atom. 1 a: the smallest particle of an element that can exist either alone or in combination an atom of hydrogen. B: the atom considered as a source of vast potential constructive or destructive energy a largely forgotten legacy of this country's conquest of the atom. The smallest particle is the election the smallest particle of a complete element is the atom but most atoms are not stable. Noble gases are far more stable on their own, Gold is also very stable but all other elements are more stable in molecules either with itself. American Elements specializes in producing high purity Aluminum Particles with the smallest possible average grain sizes for use in preparation of pressed and bonded sputtering targets and in Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD) processes including Thermal and Electron Beam (E-Beam) Evaporation, Low Temperature Organic Evaporation, Atomic Layer Deposition (ALD). The smallest particle of an element is a (n):electronneutronatom. The smallest particle of an element is a (n): electron. Report flag outlined.
What is the smallest particle of a compound?
1 Answer
Smallest Particle Of An Element Or Compound
The smallest particle of a compound which is capable of existing on its own is a Molecule- not an atom.
Explanation:
A compound can be broken down into molecules. To be specific,a molecular compound can be broken down into molecules and ionic compounds can be broken down into units,which you can consider as molecules of an ionic compound. The smallest particle of a compound capable of existing on its own is a Molecule-not a atom. An atom is the smallest particle capable of existing on its own of an ELEMENT. And yes,atoms can be further broken down into electrons,neutrons and protons.
Electrons are indivisible.
Protons and neutrons can be further divided into quarks.
Quarks are indivisible.
Since quarks and electrons are indivisible,they are called fundamental units. Note: Quarks,electons,protons,neutrons are incapable of individual existance.
Related questions
As we all know that an element is made up of an atom. As a result of this, atom is also commonly held as the smallest particle of an element. However, it was discovered in 1911 by Ernest Rutherford that an atom itself has different parts namely, electrons, protons, and neutrons. Moreover, there may also be a further division of these parts. This even more diminutive particle is called a quark.
To provide you with more details, here is some more information on the smallest particles of an element.
Smallest Particle Of An Element That Retains Its Properties
#1. Electrons
An electron is the part which provides the atom with its charge. You can make an atom positively or negatively charged by changing the number of electrons. While more electrons imply a negatively charged element, less number of electrons would mean positively charged element. It is also interesting to note that electrons revolve around the nucleus of an atom and can even bond with other atoms to form a compound.
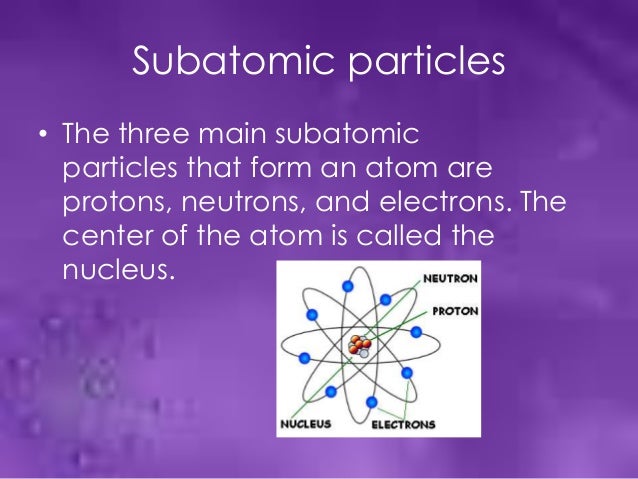
(Image Courtesy: Quora)
#2. Protons
While electrons determine the charge of an element, protons are known to determine the mass of an element. In fact, electrons have quite an inconsequential amount of mass when compared to protons. Stationed at the nucleus of an atom, the positively charged protons help in the classification of an element as an element is categorized in accordance with, the number of protons its atom contains.
(Image Courtesy: Boundless)
#3. Neutrons
Like protons, electrons are massive and are placed at the nucleus of an atom. What makes neutrons distinctive is their lack of charge. While electrons and neutrons have negative and positive charge respectively, neutrons have no charge at all. Changing the amount of neutrons has no impact on the element. However, a change in the number of neutrons is capable of making isotopes.
(Image Courtesy: Isaac's Science Blog)
#4. Quarks
Electrons and protons are further classified into different parts called quarks, which are the smallest known particle of an element. Quarks were first discovered in the year 1961 and are of six types namely, up, down, charm, strange, bottom, and top. While a quark trio can combine to form a together, a quark can also combine with an anti-quark to form a mason. However, such a combination lacks stability and may last only for a small fraction of seconds.
(Image Courtesy: Byju's)
We would love to hear from you. Share with us more details on the parts of elements. Kindly use the comment box to share your thoughts and opinions with us.
Smallest Particle Of An Element That Maintains The Properties Of That Element
(Featured Image Courtesy: Reference)
